browningmirage
Active Member
Basic chemistry says high alkaline water like ocean water 125ppm resist change to pH far greater than water with no alkalinity like precipitation does it not? With such a minuscule change showing in the precipitation then there would be no noticeable change to the oceans when considering volume of oceans and high alkalinity compared to vapour or rain with no alkalinity.
I have never said acid rain was all caused from factories in the past. I was claiming it was mainly from large volcanoes and that is why the pH has risen back to around 5.6 now. Which is considered natural before pollution. Large eruptions dwarf the pollution from civilization. That is why the pH is so high now because we know emission reductions have now gone to 0. The factories spin was GLG.
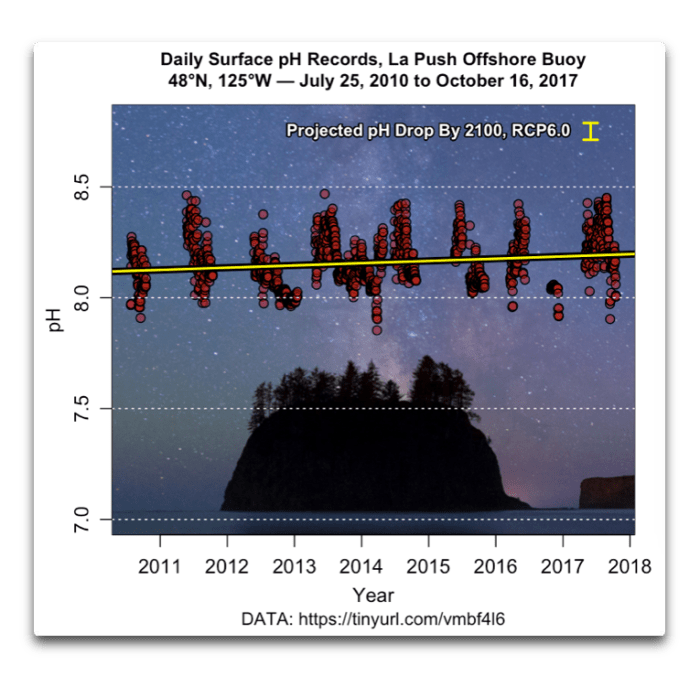
You never did comment on what you thought was going on in the last graph I posted. It was the pH readings from the LaPush?
You should cite a source saying that eruptions are bigger emitters than civilization. There have been none in the past 100 years that would even come close.