fishbadger
Active Member
Stupid headline.
But it is real.
Deflecting doesn't make it not real.
fb
But it is real.
Deflecting doesn't make it not real.
fb
Stupid headline.
But it is real.
Deflecting doesn't make it not real.
fb
substance of the research.
The article is from CNN, that is their motto. People have posted the underlying research. It is unfortunate that many choose to attack the reporting instead of the substance of the research.
Where did everyone go here? I mention a fact proving that atmospheric co2 can not be acidifying the oceans. A few people respond by attempting to discredit me but do not respond to the substance of my research. I then provided the link which validates my comments and now there is a disappearing act. Surely with all of their knowledge put together they could equal at least high school chemistry level and explain to me how my findings could be incorrect??
I dont think that's fact...its known fact that CO2 dissolves in water, making it more acidic. Long term trends of increasing pH prove nothing unless you have concentrations of the other molecules contributing to acid. Can you present us the data on all of the constituents, or do we only have pH of rainwater?
Attached is a rain chemistry report which does list some of the other elements contributing to precipitation pH. Not sure if you looked at the video I posted? The Saturna island reports does also list other elements and concentrations which would effect pH.
I by no way dispute the fact of co2 absorbing into water causes a drop in pH. How is it possible for the pH trend to be opposite between the two?
The paper you just posted explains why the two trends are going in different directions. It's the SO2 and the NOx.
We know there is a problem with putting SO2 and NOx into the atmosphere that's why we have changed regulations, over the years, to get it out of our byproducts of combustion. Fossil fuel power plants need to scrub it out. Lower the sulfur in diesel fuel to the ultra low sulfur content specification. Reduce global marine fuel sulfur content from 5% to 1%, a new regulation that kicked in as of Jan 2020. All these actions reduce the content of acid forming components that create acid rain.[/
I already know what caused the extreme acid rain in the past. It is obvious that the decrease in sulphate and nitrogen is why the rain is not as acidic as back in the 1990’s. I did not see any explanation as to how the week carbonic acid has the ability to acidify the oceans but not the less alkaline rain and fresh waters? Isn’t pH 5.6 supposed to be considered “clean” rain. If Co2 from the air is creating enough effect to acidify the oceans then we would see it effecting the precipitation which has zero acid neutralizing capability which we are not.
I think you are grasping a bit. Rain interacting with CO2 in the atmosphere, creating a weakly acidic rain is a different effect than C02 from the atmosphere interacting with slightly basic ocean water and driving the pH down.
From a biological perspective, animals are adapted to a set of conditions. Anything moving it beyond the realm of natural variability will have an impact on those animals.
The original paper could have used a bigger sample size, and there were other issues to consider. But that doesnt mean the effects weren't real. I think it needs more work, but it fits with known cause/effect, so it shouldn't be dismissed outright.
I forgot to mention thank you for the respectful interaction. Lets keep this discussion going. I know there is truth out there somewhere but for me it will take UNBIASED FACTS to convince me co2 is ruining the world and not hype from environmental activism.
If you want to register the factual change of pure rainwater pH you must buy a decent pH meter and do away with your rough colorimeter. It's a simple chemical equilibrium calculation that will show you that the pH of rainwater has indeed dropped because of the recent increase in CO2 in the atmosphere. But the pH change will be smaller than the 0.5 or 1 pH increments that you can read with your colorimeter.
Also, it is not the slight decrease in rainwater pH that is decreasing the pH of the ocean. Only in very isolated coastal bays with large freshwater influx (Fraser mouth, SOG) freshwater pH will have a measurable impact on ocean pH. 25% of atmospheric CO2 is directly absorbed by the ocean. Higher atmospheric CO2 plus less CO2 uptake on land (less plants/forests) have changed the CO2 flux (read up on this concept) and lead to increased CO2 absorption in the ocean. Oceans used to give up CO2 into atmosphere before human impact; now through fossile burning plus deforestation it's reversed and the ocean takes in CO2 - thus the ocean pH drops. So much for getting you on the right track. Hope this helps in your search.
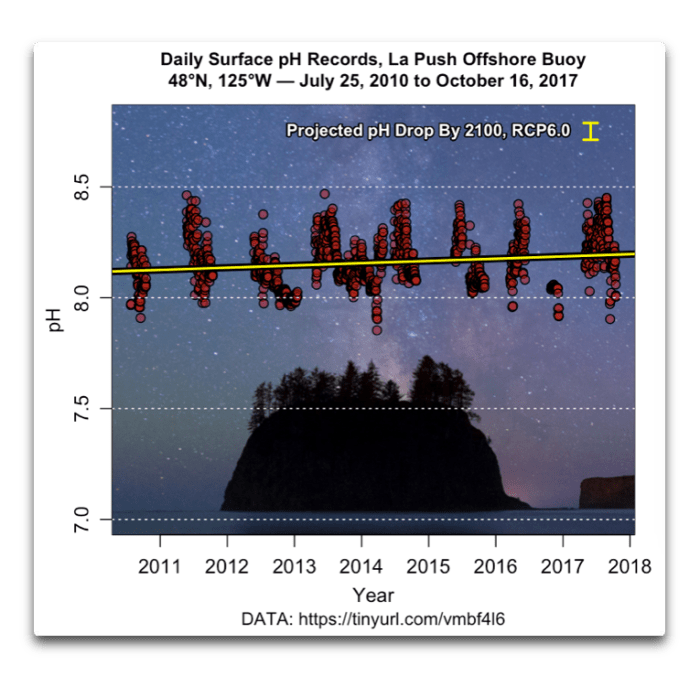
If you want to register the factual change of pure rainwater pH you must buy a decent pH meter and do away with your rough colorimeter. It's a simple chemical equilibrium calculation that will show you that the pH of rainwater has indeed dropped because of the recent increase in CO2 in the atmosphere. But the pH change will be smaller than the 0.5 or 1 pH increments that you can read with your colorimeter.
Also, it is not the slight decrease in rainwater pH that is decreasing the pH of the ocean. Only in very isolated coastal bays with large freshwater influx (Fraser mouth, SOG) freshwater pH will have a measurable impact on ocean pH. 25% of atmospheric CO2 is directly absorbed by the ocean. Higher atmospheric CO2 plus less CO2 uptake on land (less plants/forests) have changed the CO2 flux (read up on this concept) and lead to increased CO2 absorption in the ocean. Oceans used to give up CO2 into atmosphere before human impact; now through fossile burning plus deforestation it's reversed and the ocean takes in CO2 - thus the ocean pH drops. So much for getting you on the right track. Hope this helps in your search.
Ken, my sources are basic chemistry. As rain passes through an atmosphere where the CO2 concentration has risen from less than 300 ppm 200 years ago to over 400 ppm today the chemical equilibrium dictates that the rain water pH drops. It has to, this is a scientific law. But we are talking a few hundreds of 1 pH. Don't tell me you can see the difference between 5.60 and 5.52 on your colorimeter, that's bs. What you are seeing in the studies your are looking at are other factors that are having a much greater and profound impact on rainwater pH. You mentioned yourself human induced acid rain effects that where prevalent in past decades anywhere near industrial air pollution. In our western world some of these impacts have been mitigated by better emission control technology. I am sure if you measured the rainwater pH near a chinese or russian factory you could see pH of 5 or less still today.